CQL Studio
An integrated web application suite for developing, testing, and publication of CQL (Clinical Quality Language) and FHIR-based artifacts using classical IDE concepts and modern AI-assisted drafting.
Planned Key Features
Integrated Development Environment
Full-featured IDE with syntax highlighting, autocomplete, and error detection for CQL development.
Cross-Engine Testing
Comprehensive testing suite with support for multiple CQL engines and compatibility validation.
FHIR Integration
Seamless integration with FHIR servers for data retrieval and resource management.
AI-Assisted Development
Optional AI-powered assistance for CQL drafting and code generation.
Results Analysis
In-depth analysis and visualization of test results with detailed reporting.
Docker Ready
Easy deployment with Docker containers for consistent development and production environments.
An IDE for CQL and FHIR
Explore the powerful features and intuitive interface of CQL Studio through these screenshots.

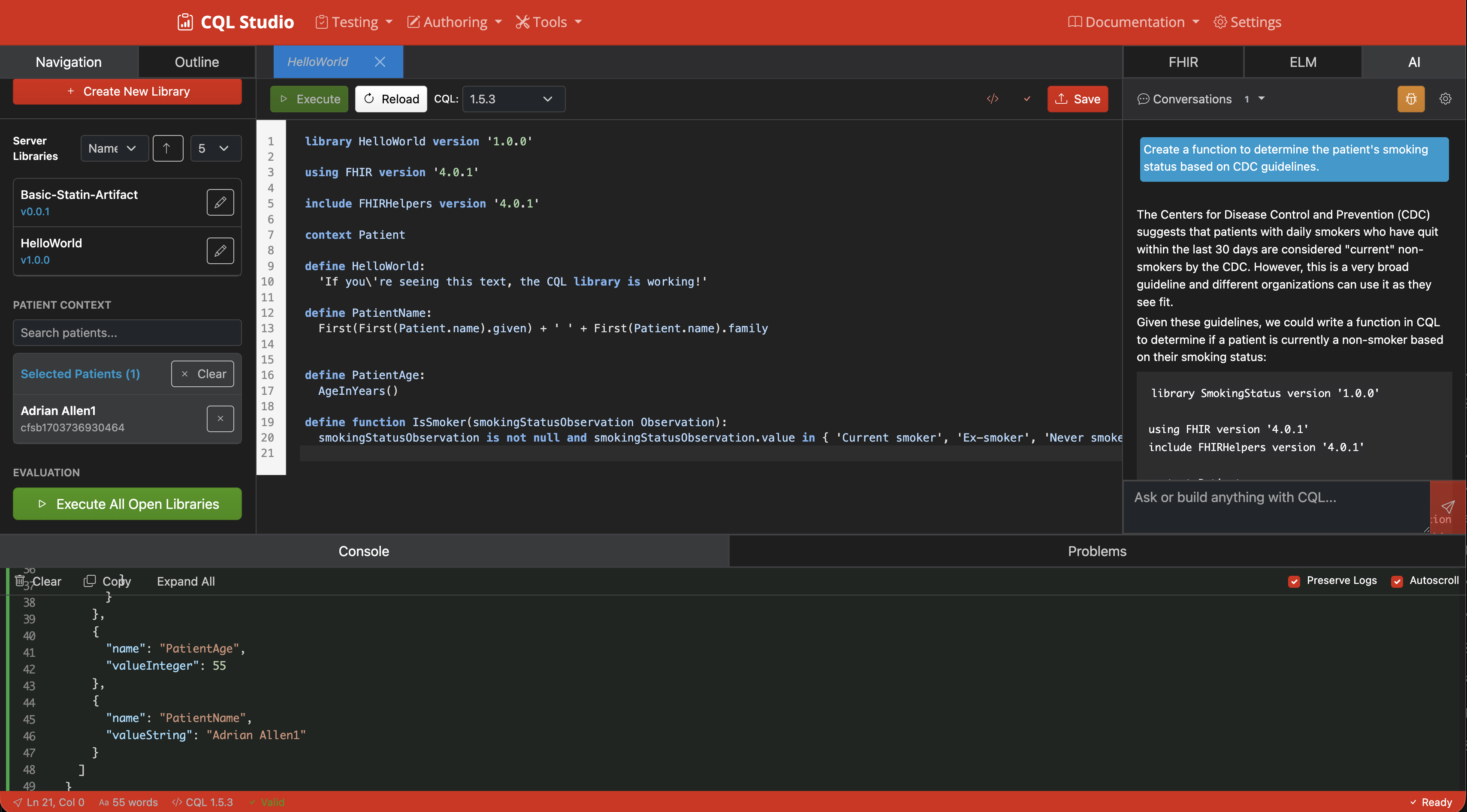
CQL IDE with Execution
Full-featured integrated development environment with syntax highlighting and execution capabilities.
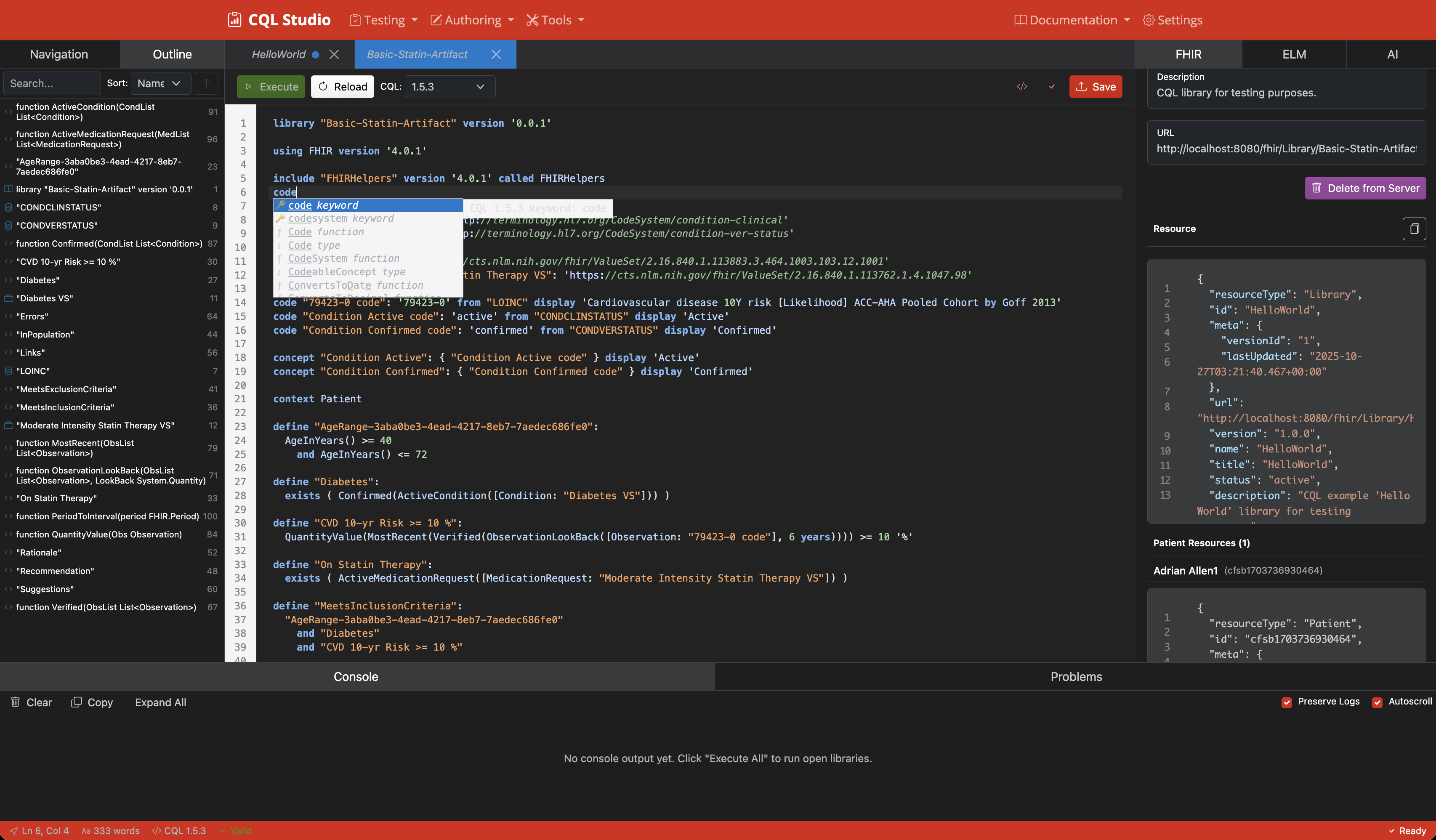
Code Outline & Navigation
Advanced code navigation with outline view and intelligent code structure analysis.
CQL Engine Testing and Results
Comprehensive testing capabilities and result analysis tools for CQL engines.
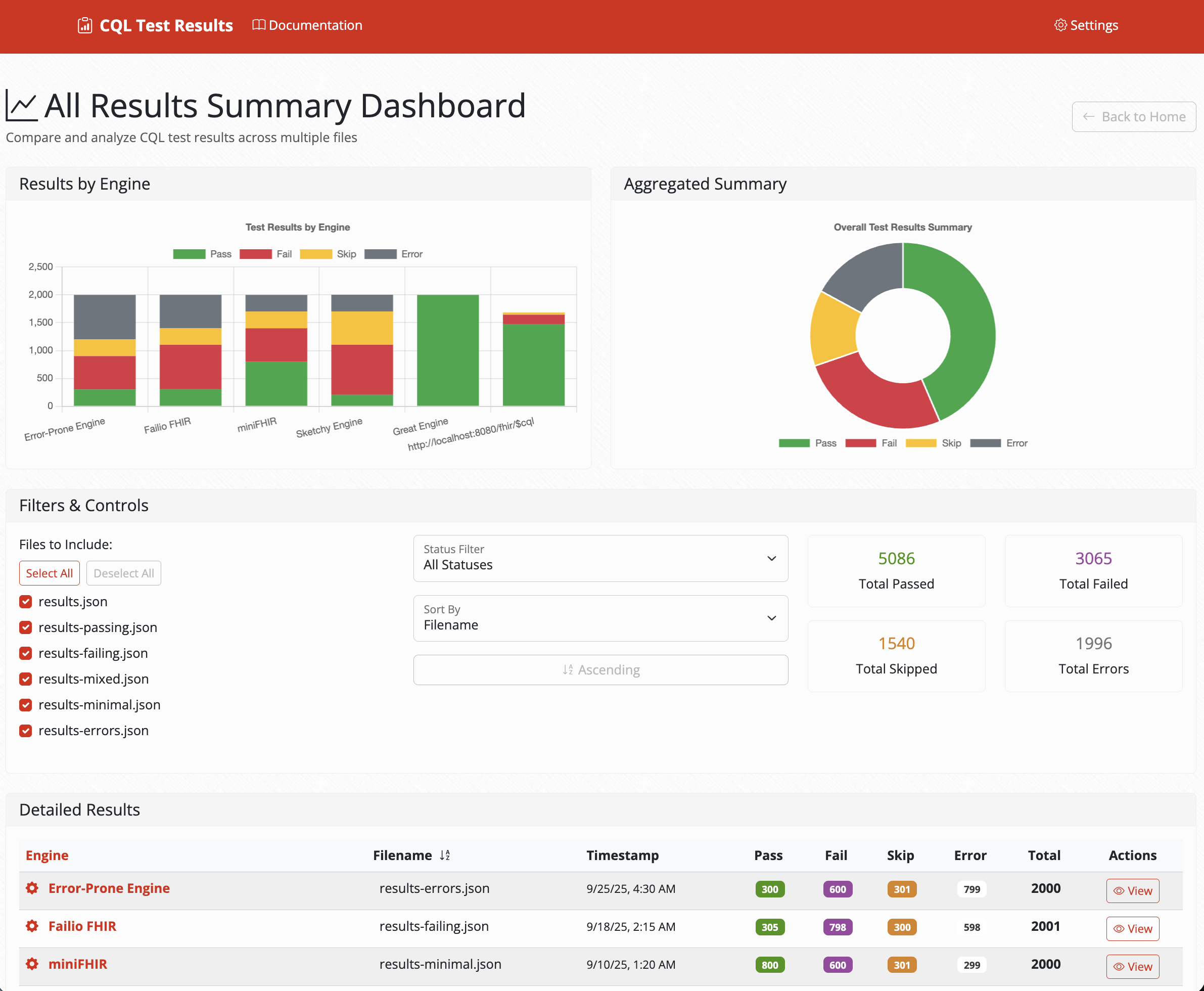
Cross-Engine Comparison Dashboard
Comprehensive overview of test results across multiple CQL engines with detailed metrics and visualizations.
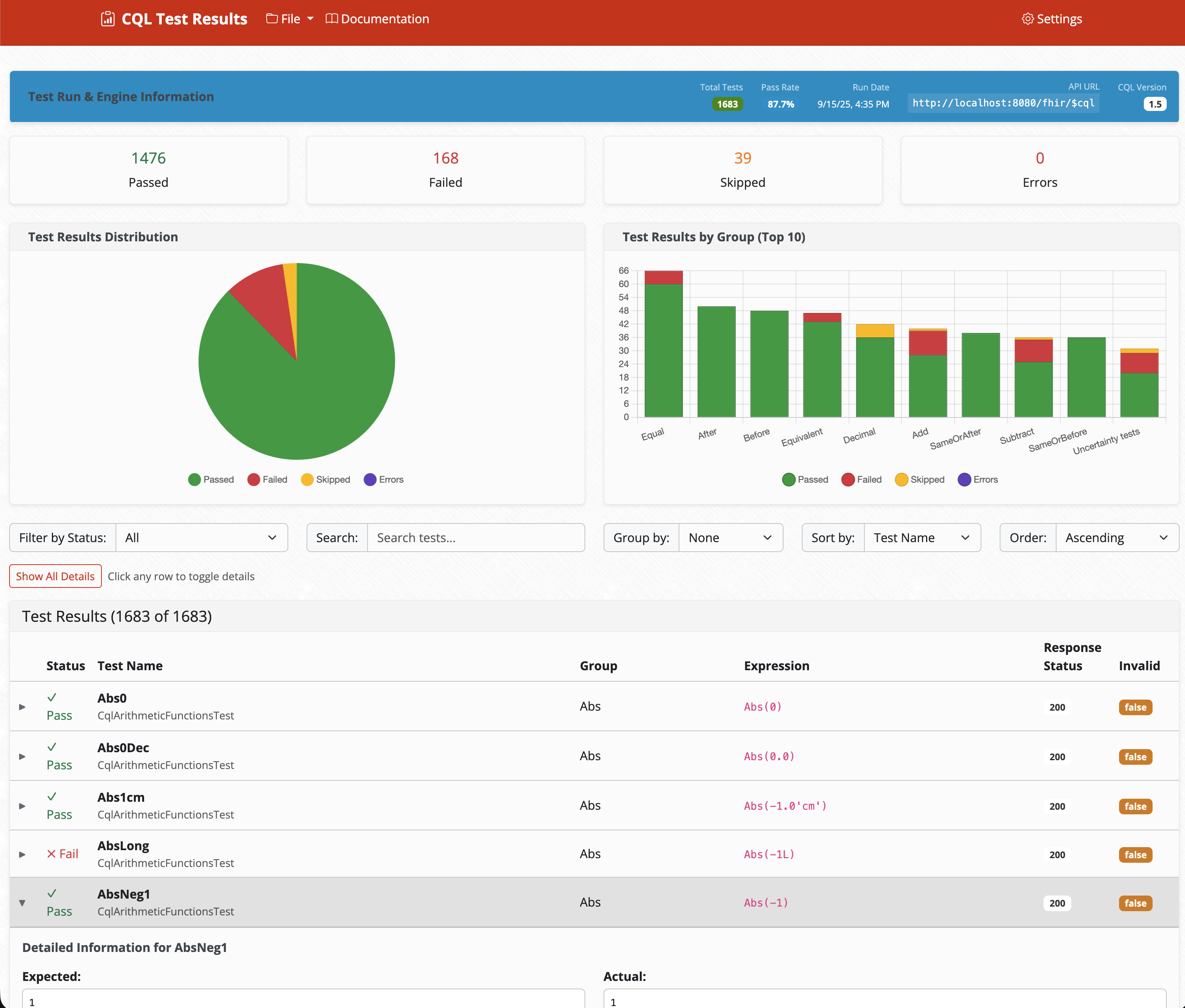
In-Depth Result Review
Detailed analysis of test results with filtering, sorting, and comprehensive reporting capabilities.
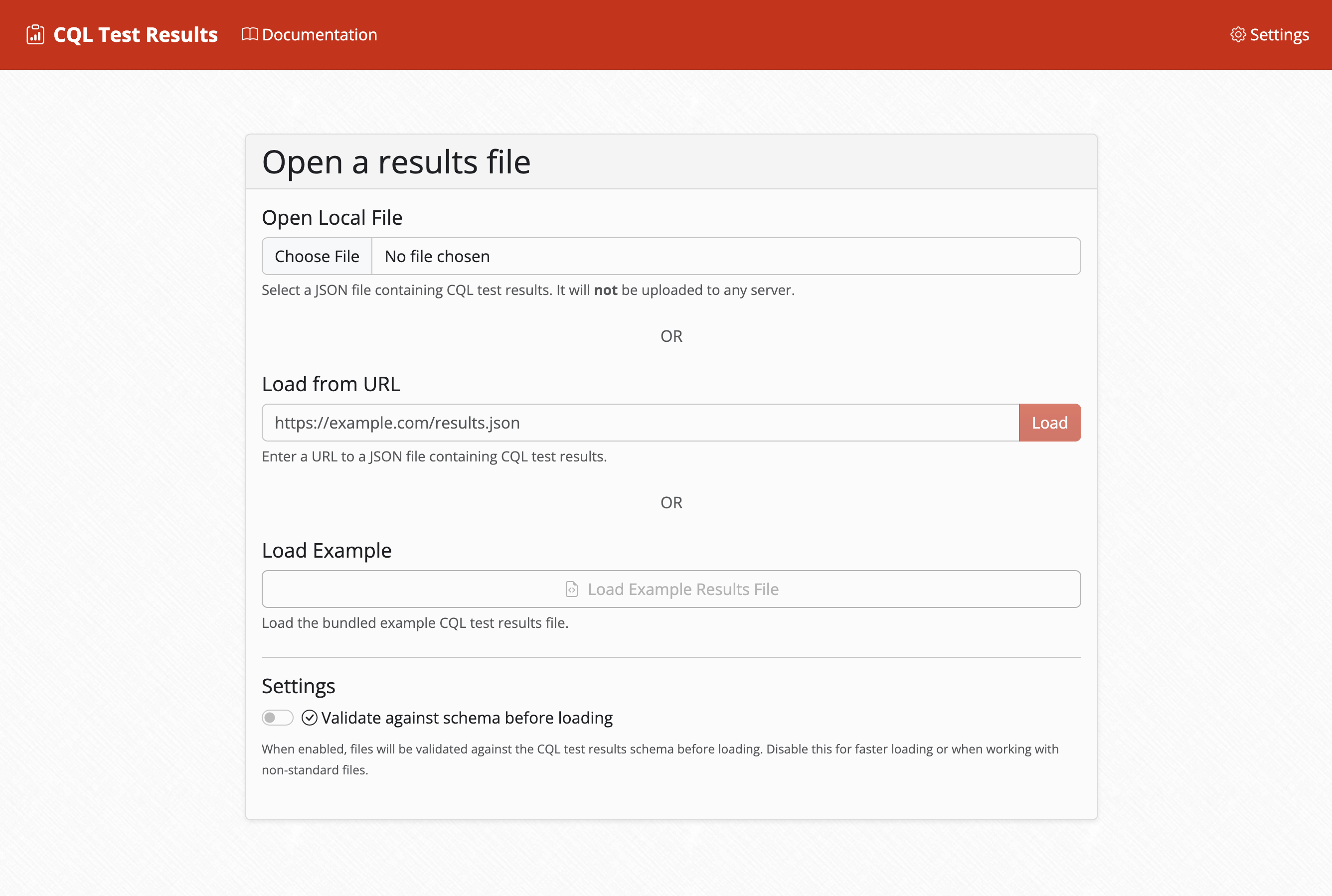
Flexible Data Loading
Multiple data loading options with support for various formats and deep-linking capabilities.
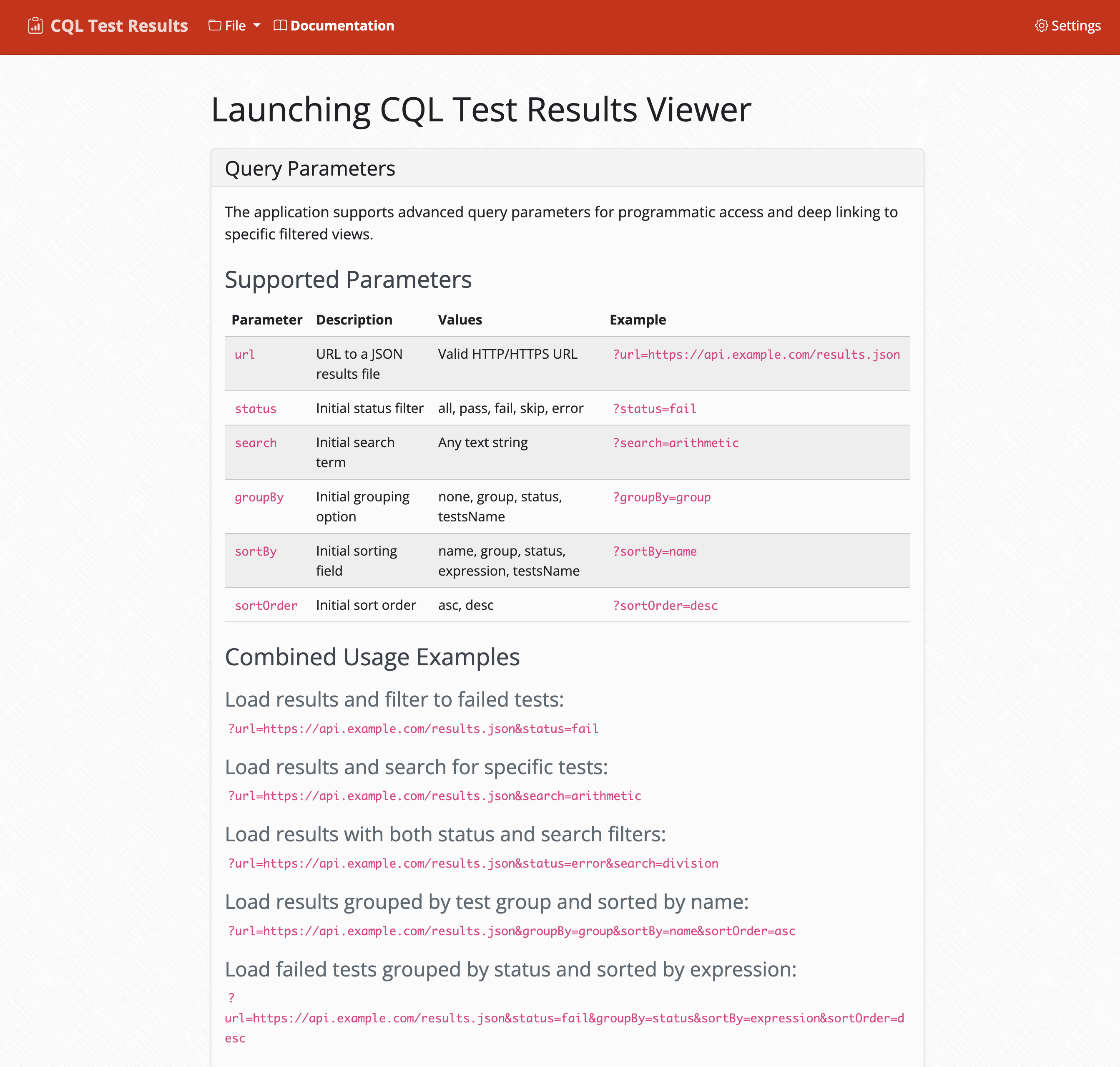
Integrated Documentation
Built-in documentation and help system with deep-linking support for seamless navigation.